Independent Research
-
36
Wei Zhao, En-Ze Lin, Ke-Zhi Chen, Yu-Wen Sun and Bi-Jie Li* Diastereo- and enantioselective 1,3-hydrofunctionalization of trisubstituted alkenes by a directing relay. Nat. Chem. 2025,.
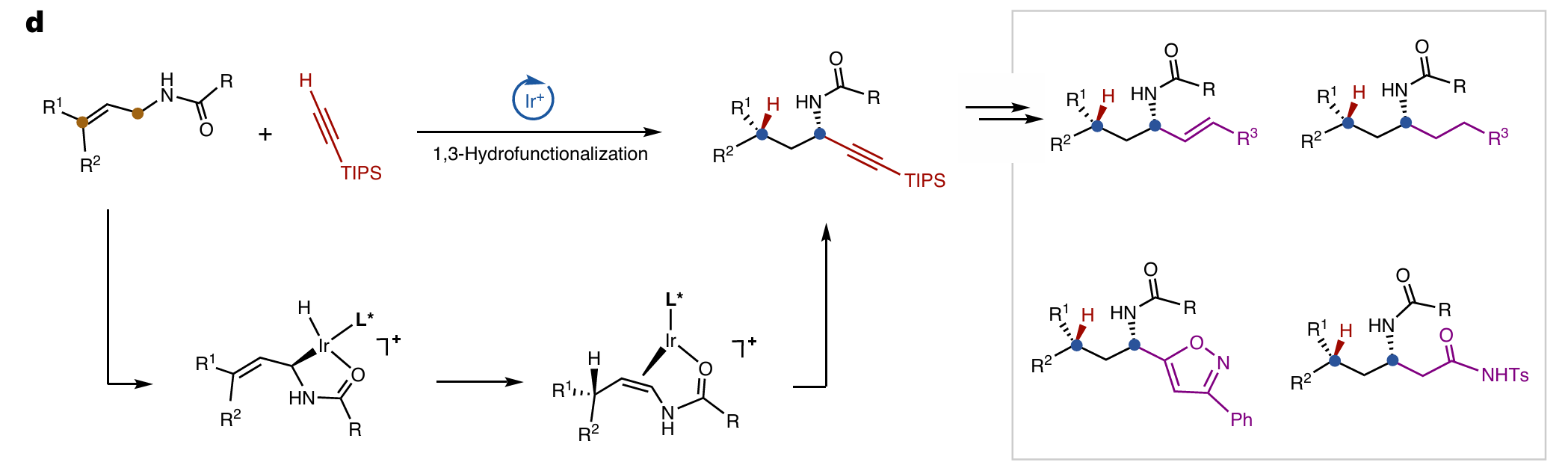
-
35
Yu-Wen Sun, Hao-Tian Tan, Sheng-Nan Sun, and Bi-Jie Li* Iridium-Catalyzed Asymmetric β-Selective Hydroamination of Enamides for the Synthesis of 1,2-Diamines. Angew. Chem. Int. Ed. 2025, e202507200.
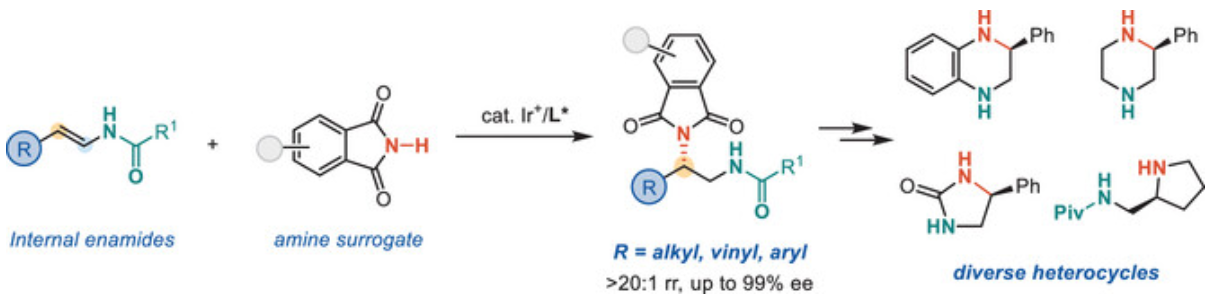
-
34
En-Ze Lin, Wei Zhao, Jun-kai Shi, Yu-Wen Sun, Xianrui Xiong, Xiaotian Qi, Xin Sun*, and Bi-Jie Li* Construction of Nonadjacent Stereocenters Through Iridium-Catalyzed Desymmetric Hydroheteroarylation of Cyclopentenes. Angew. Chem. Int. Ed. 2025, e202501641.
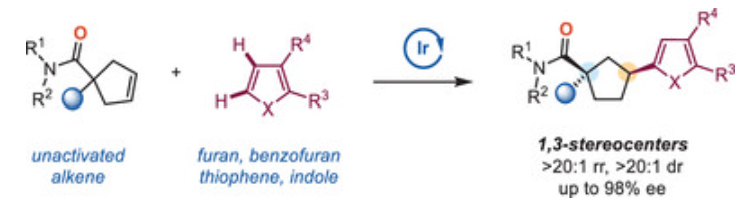
-
33
An-Zhen Li, Xiongbo Wang, Shuwei Li, Bo-Jun Yuan, Xi Wang, Ruo-pu Li, Liang Zhang, Bi-Jie Li*, and Haohong Duan* Direct Electrooxidation of Ethylene to Ethylene Glycol over 90% Faradaic Efficiency Enabled by Cl– Modification of the Pd Surface. J. Am. Chem. Soc. 2025, 147, 12, 10493–10503.
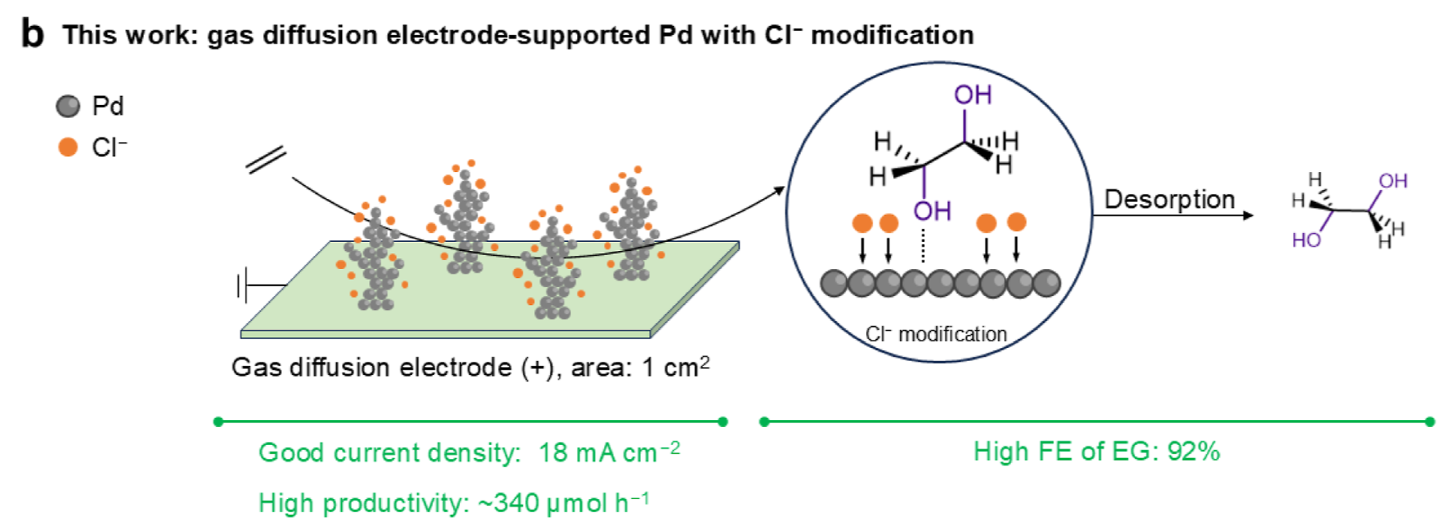
-
32
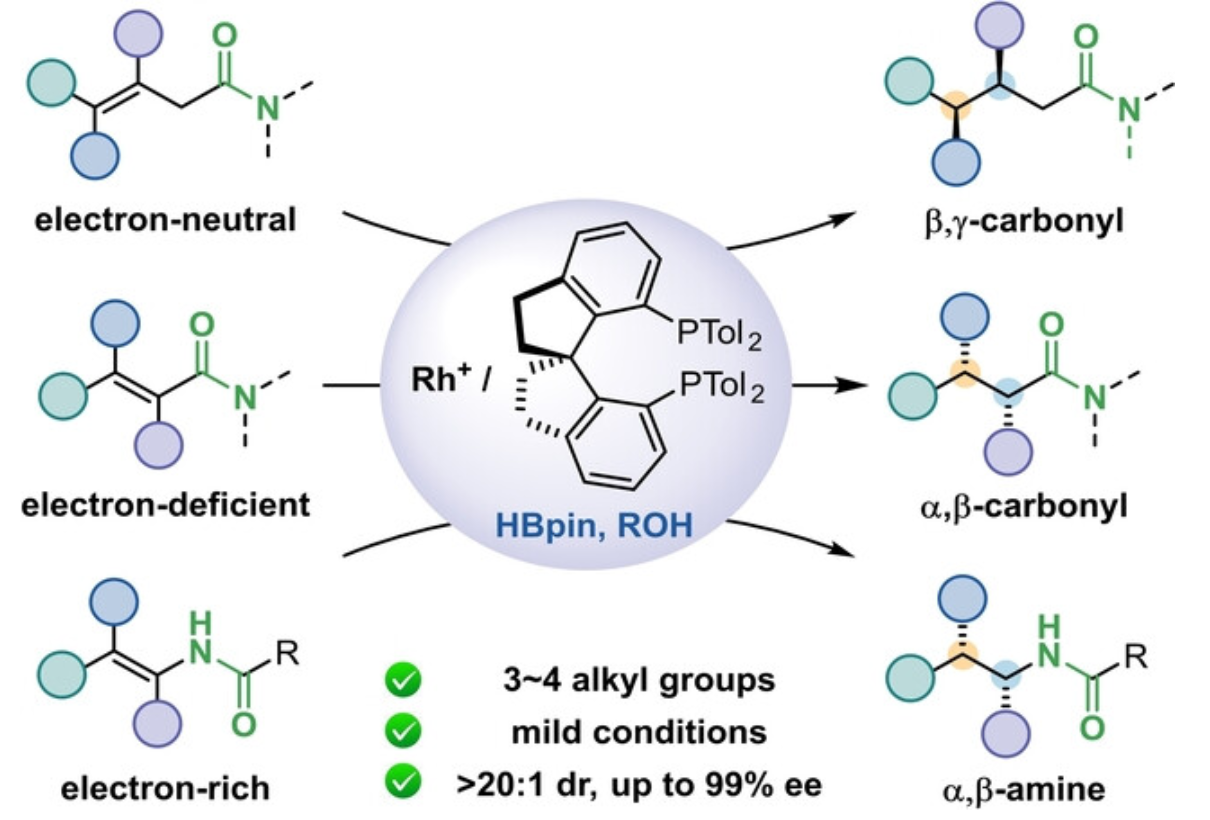
Hou-Xiang Lu, Shou-Lin Lu, and Bi-Jie Li* Amide-Directed Highly Enantioselective Hydrogenation of Diverse Acyclic Multisubstituted Alkenes Under Mild Conditions. Angew. Chem. Int. Ed. 2025, e202422698.
-
31
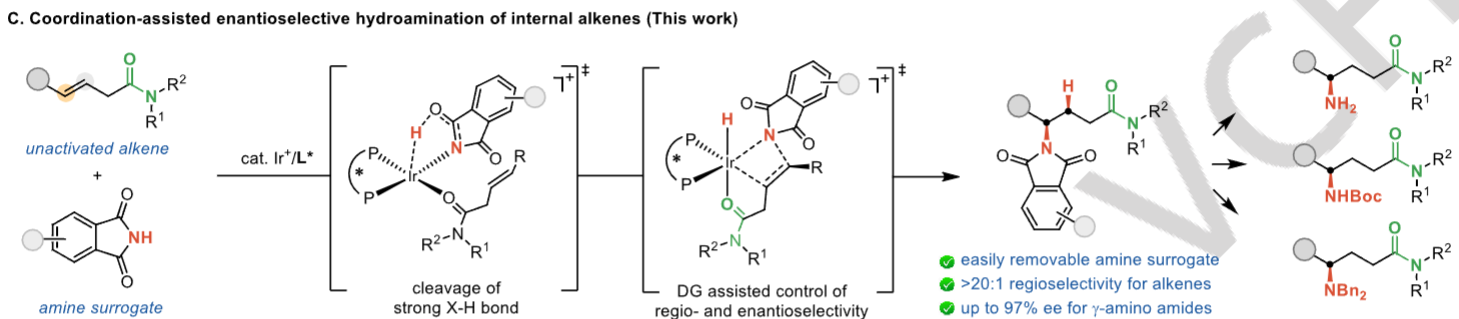
Yu-Wen Sun, Xin Sun, Hao-Tian Tan and Bi-Jie Li*, Synthesis of γ-Amino Amides by Iridium-Catalyzed Enantioselective Hydroamination of Internal Alkenes Directed by an Amide. Angew. Chem. Int. Ed. 2024, 202422944.
-
30
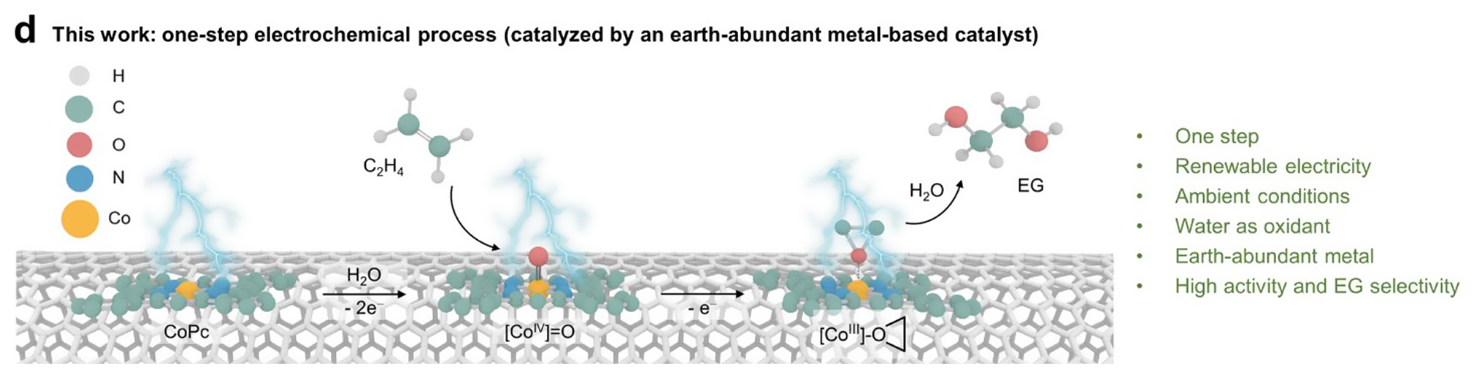
An-Zhen Li§, Bo-Jun Yuan§, Ming Xu, Ye Wang, Chunyu Zhang, Xiongbo Wang, Xi Wang, Jing Li, Lirong Zheng, Bi-Jie Li*, and Haohong Duan* One-Step Electrochemical Ethylene-to-Ethylene Glycol Conversion over a Multitasking Molecular Catalyst. J. Am. Chem. Soc. 2024, 146, 8, 5622–5633.
-
29
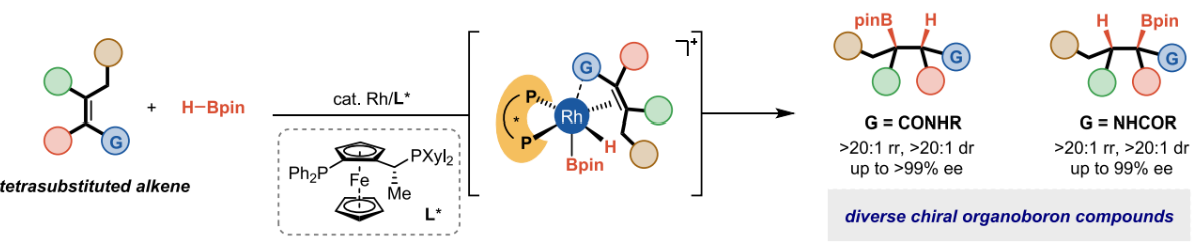
Hou-Xiang Lu§, Cheng Wang§, Tao-tao Gao, En-Ze Lin, Shou-Lin Lu, Xin Hong* and Bi-Jie Li* Rhodium-Catalyzed Highly Enantioselective Hydroboration of Acyclic Tetrasubstituted Alkenes Directed by an Amide. J. Am. Chem. Soc. 2024, 146, 23, 16194–16202.
-
28
Xin Sun§, Peng-Chao Gao§*, Yu-Wen Sun, and Bi-Jie Li* Amide-Directed, Rhodium-Catalyzed Regio- and Enantioselective Hydroacylation of Internal Alkenes with Unfunctionalized Aldehydes. J. Am. Chem. Soc. 2023, 146, 1, 723–732.
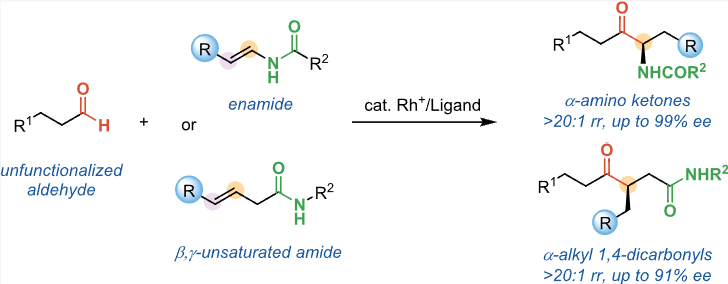
-
27
Meng Jin§, An-Zhen Li§, Ye Wang, Jing Li, Hua Zhou, Bi-Jie Li* and Haohong Duan*, Electrosynthesis of N,N-dimethylformamide from market-surplus trimethylamine coupled with hydrogen production. Green Chem. 2023, 25, 5936–5944.

-
26
Wei Zhao, and Bi-Jie Li*, Directing Group Repositioning Strategy Enabled Site- and Enantioselective Addition of Heteroaromatic C–H Bonds to Acyclic Internal Alkenes. J. Am. Chem. Soc. 2023, 145, 12, 6861–6870.

-
25
Wen-Wen Zhang, and Bi-Jie Li*, Enantioselective Hydrosilylation of β,β-Disubstituted Enamides to Construct α-Aminosilanes with Vicinal Stereocenters. Angew. Chem. Int. Ed. 2023, 62, e202214534.

-
24
Wei Zhao, Hou-Xiang Lu, Wen-Wen Zhang and Bi-Jie Li*, Coordination Assistance: A Powerful Strategy for Metal-Catalyzed Regio- and Enantioselective Hydroalkynylation of Internal Alkenes. Acc. Chem. Res. 2023, 56, 3, 308–321.

-
23
陆候祥, 李必杰*, 过渡金属催化的内烯烃不对称硼氢化. 有机化学 2022, 42, 3167−3182.

-
22
Xin Sun, En-Ze Lin, and Bi-Jie Li*, Iridium-Catalyzed Branch-Selectiveand Enantioselective Hydroalkenylation of α‑Olefins through C−H Cleavage of Enamides. J. Am. Chem. Soc. 2022, 144, 17351−17358.

-
21
Wei Zhao, Ke-Zhi Chen, An-Zhen Li and Bi-Jie Li*, Remote Stereocenter through Amide-Directed, Rhodium-Catalyzed Enantioselective Hydroboration of Unactivated Internal Alkenes. J. Am. Chem. Soc. 2022, 144, 29, 13071–13078.

-
20
Zi-Xuan Wang, Peng-Chao Gao, En-Ze Lin and Bi-Jie Li*, Stereodefined Skipped Dienes through Iridium-Catalyzed Formal Addition of Tertiary Allylic C−H Bonds to Alkynes. Angew. Chem. Int. Ed. 2022, 61, e202200075.

-
19
Xin Sun, and Bi-Jie Li*, Acyclic Quaternary Carbon Stereocenters through Transition-Metal-Catalyzed Enantioselective Functionalization of Unsaturated Hydrocarbons. Synthesis, 2022, 54(09): , 2103-2118
-
18
Zi-Xuan Wang, and Bi-Jie Li*, Iridium-Catalyzed Regiodivergent and Enantioselective Hydroalkynylation of Unactivated 1,1-DisubstitutedAlkenes. Angew. Chem.Int. Ed., 2022, e202201099.

-
17
Peng-Chao Gao, Zi-Xuan Wang, and Bi-Jie Li*, Hydroalkynylation of Internal Alkenes Directed by an Oxime. Org. Lett.. 2021, 23, 9500-9504.

-
16
Wen-Wen Zhang, and Bi-Jie Li*, Iridium-catalyzed enantioselective hydroalkynylation via alkene isomerization. Tetrahedron Letters. 2021, 73, 153108.

-
15
Su-Lei Zhang§, Wen-Wen Zhang§, and Bi-Jie Li*, Ir-Catalyzed Regio- and Enantioselective Hydroalkynylation of Trisubstituted Alkene to Access All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc.. 2021, 143, 9639−9647.

-
14
Tao-Tao Gao, Hou-Xiang Lu, Peng-Chao Gao, and Bi-Jie Li*, Enantioselective synthesis of tertiary boronic esters through catalytic asymmetric reversed hydroboration. NATURE COMMUNICATIONS. 2021, 12, 3776.

-
13
Xin Sun, Xiao-Yan Bai,* An-Zhen Li, and Bi-Jie Li*, Iridium-Catalyzed Asymmetric Hydroalkenylation of Norbornene Derivatives. Organometallics. 2021, 40, 14, 2182-2187.

-
12
Xin Sun, Wei Zhao, Bi-Jie Li*, Iridium-catalyzed, ligand-controlled directed alkynylation and alkenylation of arenes with terminal alkynes. Chem. Commun. 2020, 56, 1298-1301.

-
11
Wen‐Wen Zhang, Su‐Lei Zhang, Bi‐Jie Li*, Highly Enantioselective Synthesis of Propargyl Amide with Vicinal Stereocenters through Ir-Catalyzed Hydroalkynylation. Angew. Chem. Int. Ed. 2020, 59, 6874.

-
10
Bai, X. -Y.‡; Zhao, W.‡; Sun, X.; Li, B.-J.*, Rhodium-Catalyzed Regiodivergent and Enantioselective Hydroboration of Enamides. J. Am. Chem. Soc. 2019, 141, 19870-19878.

-
09
Wang, Z. X.; Bai, X. Y.; Li, B.-J.*, Metal-Catalyzed Substrate-Directed Enantioselective Functionalization of Unactivated Alkenes. Chin. J. Chem. 2019, 37, 1174.
-
08
Wang, Z.-X; Li, B.-J.*, Construction of Acyclic Quaternary Carbon Stereocenters by Catalytic Asymmetric Hydroalkynylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 9312.

-
07
Gao, T.-T.; Zhang, W.-W.; Sun, X.; Lu, H.-X.; Li, B.-J.*,Stereodivergent Synthesis through Catalytic Asymmetric Reversed Hydroboration. J. Am. Chem. Soc. 2019, 141, 4670-4677.

-
06
Chen, Y.; Wang, Z.-X.; Li, Q.*; Xu, L.-J.*; Li, B.-J.*, Iridium-catalysed conjugated alkynylation of α,β-unsaturated amide through alkene isomerization. Org. Chem. Front. 2018, 5, 1815.

-
05
Wen‐Wen Zhang, Tao-Tao Gao, Li-Jin Xu*, Bi‐Jie Li*, Macrolactonization of Alkynyl Alcohol through Rh(I)/Yb(III) Catalysis. Org. Lett. 2018, 20, 6534.

-
04
Wang, Z.-X.; Bai, X.-Y.; Li, B.-J.*, Recent Progress of Transition-Metal-Catalyzed Enantioselective Hydroalkynylation of Alkenes. Synlett 2017, 28, 509.
-
03
Bai, X. -Y.; Zhang, W. -W.; Li, Q; Li, B. -J.*, Highly Enantioselective Synthesis of Propargyl Amides through Rh Catalyzed Asymmetric Hydroalkynylation of Enamides: Scope, Mechanism, and Origin of Selectivity. J. Am. Chem. Soc. 2018, 140, 506-514.

-
02
Wang, Z.-X.; Bai, X.-Y.; Yao, H.-C.; Li, B.-J.*, Synthesis of Amides with Remote Stereocenters by Catalytic Asymmetric γ-Alkynylation of α,β-Unsaturated Amides. J. Am. Chem. Soc. 2016, 138, 14872.

-
01
Bai, X. -Y.‡; Wang, Z. -X.‡; Li, B. -J.*, Iridium-Catalyzed Enantioselective Hydroalkynylation of Enamides for the Synthesis of Homopropargyl Amides. Angew. Chem. Int. Ed. 2016, 55, 9007-9011.

Publications during Ph.D. Study and Postdoctoral ResearchFirst-Author Research Publications |
|
| 1. | Z.-J. Shi*, B.-J. Li, X.-B. Wan, J. Cheng, Z. Fang, B. Cao, C.-M. Qin, Y. Wang, Suzuki-Miyaura Coupling Reaction by Pd(II)-Catalyzed Aromatic C-H Bond Activation Directed by an N-Alkyl Acetamino Group, Angew. Chem. Int. Ed. 2007, 46, 5554-5558. |
| 2. | B.-J. Li, S.-L. Tian, Z. Fang, Z.-J. Shi*, Multiple C-H Activations to Construct Biologically Active Molecules in a Process Completely Free of Organohalogen and Organometallic Components, Angew. Chem. Int. Ed. 2008, 47, 1115-1118. |
| 3. | B.-J. Li, Y.-Z. Li, X.-Y. Lu, J. Liu, B.-T. Guan, Z.-J. Shi*, Cross-Coupling of Aryl/Alkenyl Pivalates with Organozinc Reagents through Nickel-Catalyzed C-O Bond Activation under Mild Reaction Conditions, Angew. Chem. Int. Ed. 2008, 47, 10124-10127. |
| 4. | Y.-Z. Li†, B.-J. Li†, X.-Y. Lu, S. Lin, Z.-J. Shi*, Cross Dehydrogenative Arylation (CDA) of a Benzylic C-H Bond with Arenes by Iron Catalysis, Angew. Chem. Int. Ed. 2009, 48, 3817-3820. (†Equal contribution) |
| 5. | B.-J. Li, L. Xu, Z.-H. Wu, B.-T. Guan, C.-L. Sun, B.-Q. Wang, Z.-J. Shi*, Cross-Coupling of Alkenyl/Aryl Carboxylates with Grignard Reagent via Fe-Catalyzed C-O Bond Activation, J. Am. Chem. Soc. 2009, 131, 14656-14657. |
| 6. | B.-J. Li, Z.-J. Shi*, An unexpected palladium migration from vinylic sp2 carbon to allylic sp3 carbon, Chin. Sci. Bull. 2010, 55, 2807–2810. |
| 7. | B.-J. Li, Z.-J. Shi*, Ir-Catalyzed Highly Selective Addition of Pyridyl C–H Bonds to Aldehydes Promoted by Triethylsilane, Chem. Sci. 2011, 2, 488-493. |
| 8. | B.-J. Li, H.-Y. Wang, Q.-L. Zhu; Z.-J. Shi*, Rhodium/Copper-Catalyzed Annulation of Benzimides with Internal Alkynes: Indenone Synthesis through Sequential C-H and C-N Cleavage, Angew. Chem. Int. Ed. 2012, 51, 3948-3952. |
| 9. | B. L. Tran†, B.-J. Li†, M. Driess, J. F. Hartwig*, Copper-Catalyzed Intermolecular Amidation and Imidation of Unactivated Alkanes, J. Am. Chem. Soc. 2014, 136, 2555-2563. (†Equal contribution) |
| 10. | B.-J. Li, M. Driess, J. F. Hartwig*, Iridium-Catalyzed Regioselective Silylation of Secondary Alkyl C–H Bonds for the Synthesis of 1,3-Diols, J. Am. Chem. Soc. 2014, 136, 6586-6589. |
| Non First-Author Research Publications | |
| 1. | X-B. Wan, Z.-X. Ma, B.-J. Li, K.-Y. Zhang, S.-K. Cao, S.-W. Zhang, Z.-J. Shi*, Highly Selective C−H Functionalization/Halogenation of Acetanilide, J. Am. Chem. Soc. 206, 128, 7416-7417. |
| 2. | S.-D. Yang, B.-J. Li, X.-B. Wan, Z.-J. Shi*, Ortho Arylation of Acetanilides via Pd(II)-Catalyzed C−H Functionalization, J. Am. Chem. Soc. 2007, 129, 6066–6067. |
| 3. | B.-T. Guan, Y. Wang, B.-J. Li, D.-G. Yu, Z.-J. Shi*, Biaryl Construction via Ni-Catalyzed C-O Activation of Phenolic Carboxylates, J. Am. Chem. Soc. 2008, 130, 14468-14470. |
| 4. | S.-D. Yang, C.-L. Sun, Z. Fang, B.-J. Li, Y.-Z. Li, Z.-J. Shi*, Palladium-Catalyzed Direct Arylation of (Hetero)Arenes with Aryl Boronic Acids, Angew. Chem. Int. Ed. 2008, 47, 1473-1476. |
| 5. | D.-G. Yu, M. Yu, B.-T. Guan, B.-J. Li, Y. Zheng, Z.-H. Wu, Z.-J. Shi*, Carbon-Carbon Formation via Ni-Catalyzed Suzuki-Miyaura Coupling through C-CN Bond Cleavage of Aryl Nitrile, Org. Lett. 2009, 11, 3374-3377. |
| 6. | C.-L. Sun, N. Liu, B.-J. Li, D.-G. Yu, Y. Wang, Z.-J. Shi*, Pd-Catalyzed C-H Functionalizations of O-Methyl Oximes with Arylboronic Acids, Org. Lett. 2010, 12, 184-187. |
| 7. | L. Xu, B.-J. Li*, Z.-H. Wu, X.-Y. Lu, B.-T. Guan, B.-Q. Wang, K.-Q. Zhao, Z.-J. Shi*, Nickel-Catalyzed Efficient and Practical Suzuki-Miyaura Coupling of Alkenyl and Aryl Carbamates with Aryl Boroxines, Org. Lett. 2010, 12, 884-887. |
| 8. | D.-G. Yu, B.-J. Li, S.-F. Zheng, B.-T. Guan, B.-Q. Wang, Z.-J. Shi*, Direct Application of Phenolic Salts to Nickel-Catalyzed Cross-Coupling Reactions with Aryl Grignard Reagents, Angew. Chem. Int. Ed. 2010, 49, 4566-4570. |
| 9. | Y. Li, B.-J. Li, W.-H. Wang, W.-P. Huang, X.-S. Zhang, K. Chen, Z.-J. Shi*, Rhodium-Catalyzed Direct Addition of Aryl C-H Bonds to N-Sulfonyl Aldimines, Angew. Chem. Int. Ed. 2011, 50, 2115-2119. |
| Accounts and Reviews | |
| 1. | B.-J. Li, S.-D. Yang, Z.-J. Shi*, Recent Advances in Direct Arylation via Palladium-Catalyzed Aromatic C-H Activation, Synlett. 2007, 7, 949-957. (Account). |
| 2. | B.-J. Li, D.-G. Yu, C.-L. Sun, Z.-J. Shi*, Activation of "Inert" Alkenyl/Aryl C-O Bond and Its Application in Cross-Coupling Reactions, Chem. Eur. J. 2011, 17, 1728-1759. (Review). |
| 3. | L.-M. Xu†, B.-J. Li†, Z. Yang, Z.-J. Shi*, Organopalladium(IV) Chemistry, Chem. Soc. Rev. 2010, 39, 712-733. (†Equal contribution, Review). |
| 4. | B.-J. Li, Z.-J. Shi*, From C(sp2)-H to C(sp3)-H: Systematic Studies on Transition Metal-Catalyzed Oxidative C-C Formation, Chem. Soc. Rev. 2012, 41, 5588-5598. (Review). |
| 5. | C.-L. Sun, B.-J. Li, Z.-J. Shi*, Pd-Catalyzed Oxidative Coupling with Organometallic Reagents via C-H Activation, Chem. Commun. 2010, 46, 677-685. (Feature Article). |
| 6. | D.-G. Yu, B.-J. Li, Z.-J. Shi*, Exploration of New C-O Electrophiles in Cross-Coupling Reactions, Acc. Chem. Res. 2010, 43, 1486–1495. (Account). |
| 7. | C.-L. Sun, B.-J. Li, Z.-J. Shi*, Macrolactones and Macrolactams, In Handbook of Cyclization Reactions. 2010, 2, 1055-1097. (Book Chapter). |
| 8. | C.-L. Sun, B.-J. Li, Z.-J. Shi*, Dierct C-H Transformation via Iron Catalysis, Chem. Rev. 2011, 111, 1293-1314. (Review). |
| 9. | H. Li, B.-J. Li, Z.-J. Shi*, Challenge and Progress: Palladium-Catalyzed sp3 C-H Activation, Catal. Sci. Technol. 2011, 1, 191-206. (Review). |
| 10. | D.-G. Yu, B.-J. Li, Z.-J. Shi*, Challenges in C-C Bond Formation through Direct Transformations of sp2 C-H Bonds, Tetrahedron. 2012, 68, 5130-5136. (Account). |
