Research
Metal-Catalyzed, Substrate-Directed Enantioselective Functionalization of Alkenes
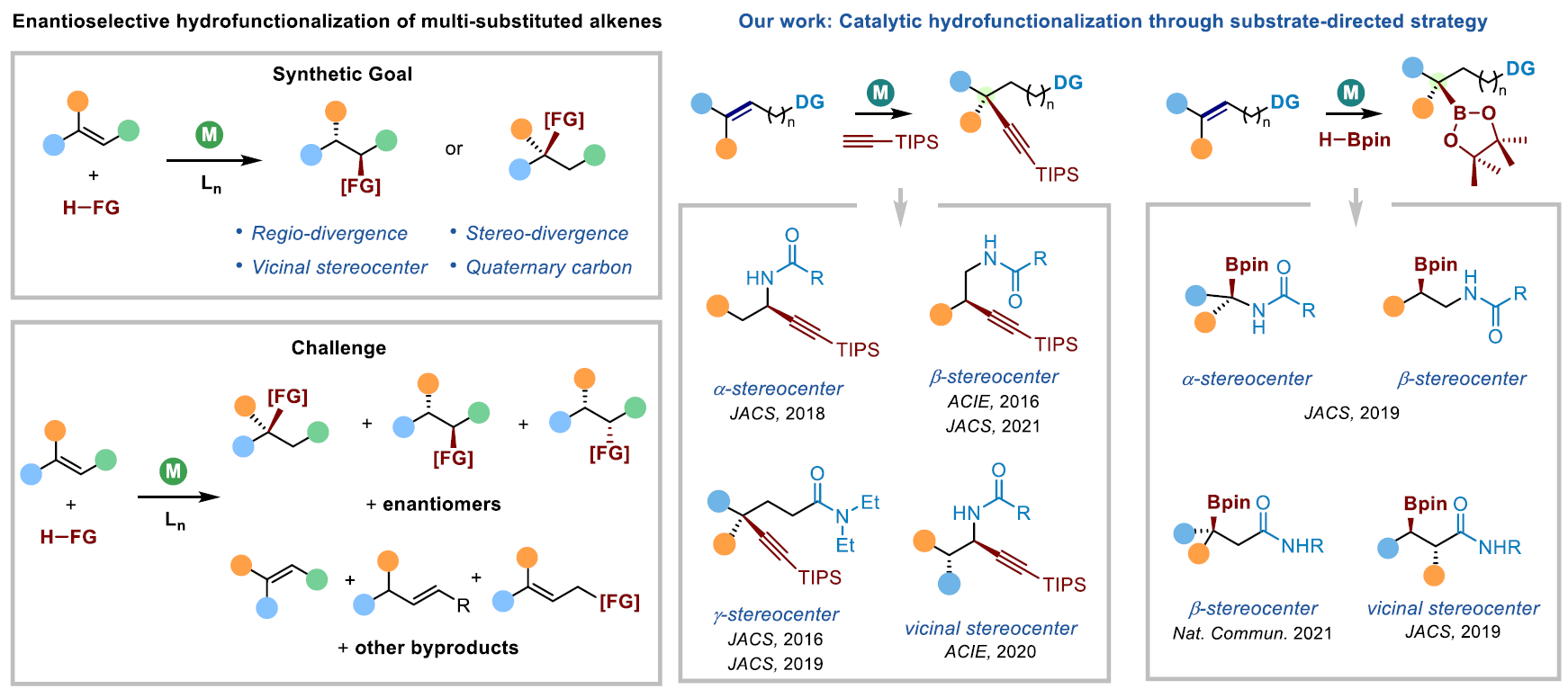
Catalytic asymmetric hydrofunctionalization of alkene enables efficient construction of chiral molecules from readily available starting materials. Asymmetric hydrofunctionalization of multiple substituted alkenes represents a significant challenge to organic chemists because this process involves the simutaneous control of regio-, diastereo-, and enantioselectivities. The key to solve this challenge is to identify novel catalyst systems to exert powerful regio- and stereo-control. Recently, by taking advantage of subsrate-directed strategy, we have developed a number of alkene functionalization methods with excellent regio-, diastereo-, and enantioselectivities. In particular, we focus on catalytic asymmetric hydroalkynylation and hydroboration of alkenes as model transformations to analyze the factors that control the selectivity.